How does pulse oximetry work?
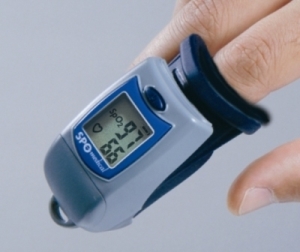
A pulse oximeter is a device used to estimate the amount of oxygen in the body.
Simply put, a pulse oximeter measures how much light haemoglobin absorbs in your blood. It does this using two different wavelengths of light, one that is preferentially absorbed by deoxygenated haemoglobin (red) and the other by oxygenated haemoglobin (near-infrared one).
The ratio of these absorbances gives a calibration curve, so the pulse ox can tell if it is all deoxygenated haemoglobin or all oxygenated (or more helpfully, somewhere in the middle!), and hence the level of saturation.
The pulse oximeter can’t record both values at once, so rapidly switches the two diodes on and off to get each signal individually. This also allows for any ambient light that might be affecting the signal too.
What is a pulse oximeter made of?
- A red bulb
- An near-infrared bulb
- A light detector
- A mini computer to process the information and calculate a number
How does it work?
When you put your finger in the pulse oximeter the absorbances of the two wavelengths change significantly. This change is what the pulse oximeter measures, and turns into numbers.
However the changes depend on a lot of factors – the thickness of your finger, the thickness of the artery, the thickness of the vein and the concentration of the haemoglobin in the blood.
Fortunately most of these issues only affect the overall absorbances, but do not affect the ratio. This means we only need generate another calibration curve to allow for these issues. Hence a pulse oximeter is calibrated using human volunteers. Of course this doesn’t mean every individual pulse oximeter is calibrated by volunteers! The volunteers are used to generate the calibration curve by comparing known saturations with the pulse oximeters reading.
The limitations of pulse oximetry
As just mentioned, pulse oximetry, although cheap, useful and quick is limited in its scope. Unfortunately moving patients seriously affect the readings, and so pulse oximetry isn’t really useful in this context. The technique also struggles when people are cold – there is not enough blood in the fingers to measure – or if they are wearing the wrong colour nail varnish – the nail varnish absorbs the light instead and gives an erroneous reading.
However, there are problems that are not solvable.
The part of haemoglobin that is absorbing all this light is the iron atom at the centre of it, where the oxygen binds to be transported around the blood. When oxygen is bound to haemoglobin it causes a change in shape and electronic structure of the haemoglobin, giving the characteristic red colour.
Oxygen however is not the only molecule to do this. Cyanide molecules, as well as carbon monoxide (amongst others), also give rise to a similar kind of binding. When either of these molecules are bound, it produces a similar colour to that of oxygenated haemoglobin, so the pulse oximeter can’t tell the difference!
A pulse oximetry reading should therefore not be trusted in cases of suspected carbon monoxide poisoning
Did you find this post interesting? Why not sign up to one of our FREE online first aid courses!