Why is Carbon Monoxide so Dangerous?
Carbon Monoxide is a deadly, odorless, gas. In this first aid blog post, we will look closer at carbon monoxide and why it is regarded as the ‘silent killer’.
Carbon monoxide is a by-product of combustion and is one of the numerous chemicals in common smoke. It is present in high concentrations in motor vehicle exhaust fumes and fumes from some types of home space healers.
Since it is colorless, odorless, and tasteless, its presence is virtually impossible to detect. Carbon monoxide binds to hemoglobin (257 times stronger than oxygen), resulting in the hemoglobin being unable to transport oxygen. This feature is what makes carbon monoxide such a deadly gas.

Patients quickly become hypoxic even in the presence of low concentrations of carbon monoxide. An alteration in the level of consciousness is the predominant sign of this hypoxia.
A cherry-red skin color or cyanosis is rarely present as a result of carbon monoxide poisoning and, therefore, cannot be used in the assessment of patients for carbon monoxide poisoning.
Pulse oximetry will remain normal – high in the presence of carbon monoxide and cannot be used to assess these patients. Some newer model pulse oximeters can now specifically measure carboxyhemoglobin levels and, if available, should be used on all persons who have the possibility of exposure to carbon monoxide.
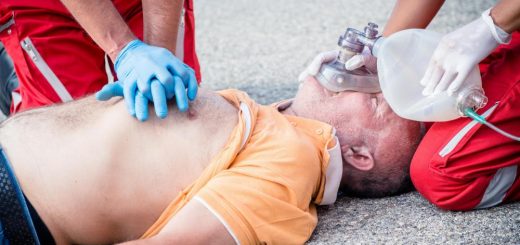
Death usually occurs because of either the brain or heart becoming starved of oxygen. Eventually, the patient will go into cardiac arrest and stop breathing normally.
Early advanced medical help is vital. If trained, first responders should administer high-flow oxygen to all patients with suspected carbon monoxide poisoning.